Which of the Following Is Not an Alkali Metal
Oxides peroxides superoxides. Which element is not an alkali metal.

Alkali Metal Definition Properties Facts Britannica
Hydrogen can show properties or transform into an alkali metal when it is exposed to extremely high pressure.

. Which of the following is not a characteristic of alkali metals alkali metals so they are metals dukan se elements which belongs to group that they belong to first group having general electronic configuration you can write that it is a of inert gas and then we are having and so. They all have 2 electrons in their valence shells They are low density solids at room temperature c. C Alkali metals are strongly reducing agents.
S Block Elements Alakali Metals MCQ Sheet 1. Chemistry S Block Elements Group 1 Elements Alkali Metals. Which of the following metal is not an alkali metal.
In nature noble gases exist as. Not to be confused with Alkaline earth metal. Which of the following is NOT an alkali metal.
Which of the following metal is not an alkali metal. Mg is one of the alkaline earth metals in Group 2 of the modern periodic table. To which group of the periodic table do the alkali metals belong.
Chemistry questions and answers. A magnesium b rubidium c sodium d caesium Answer. Note that HydrogenH is not an alkali metal.
The characteristic not related to alkali metal is. Which of the following metal is not an alkali metal. The outer shell configuration of group 1 elements is ns 1 where n is the number of its period.
This group lies in the s-block of the periodic table as all alkali metals have their outermost electron in an s-orbital. The correct option is D They are diamagnetic in nature M x y N H 3 M N H 3 x e N H 3 y. Dilute solutions of alkali metals in liquid ammonia are dark blue in colour and the main species present are ammoniated cation and solvated electrons.
I cant understand your question mate but these are all the alkali metals because they are in the first group. However the main reason why hydrogen H is not considered as an alkali metal is that it is mostly found as a gas when the temperature and pressure are normal. Magnesium is not an alkali metal because its outer shell configuration is ns 2.
Due to the large size least tendency to accept electron. Li o ion due to its small size does not form a stable lattice structure. Alkali metals are the elements of group 1.
All the alkali metals react with water with the heavier alkali metals reacting more vigorously than the lighter ones. The alkali metals consist of the chemical elements lithium Li sodium Na potassium K rubidium Rb caesium Cs and francium Fr. Which of the following is not an alkali metal.
Together with hydrogen they constitute. Which of the following is not true about alkali metals. Which of the following statements regarding alkali metals is not correct.
The characteristic not related to alkali metal is. Name the main factor which is responsible for the anomalous behaviour of lithium. Alkali metals are.
Other alkaline earth metals are Be Ca Sr Ba Ra. Hence option 1 is correct. A Alkali metals are soft and have comparatively low melting points as compared to other metals.
What is the main component of steel. All of these elements are alkali metals. The chemical name for rust is.
They all have 2 electrons in their valnce shells. Name the alkali metal which floats on water without any apparent reaction with it. The best conductor of electricity is.
B Francium is a radioactive element. Name the alkali metals which form superoxides on heating in excess of air. In the modern IUPAC nomenclature the alkali metals comprise the group 1 elements excluding hydrogen.
LithiumLi SodiumNa PotassiumK RubidiumRb CesiumCs and FranciumFr. Group 1 on extreme left position contains alkali metalsLi Na K Rb Cs and Fr. Properties of alkali metal.
The reaction of alkali metals with oxygen produce. Correct option is D D All of them is alkali metals. The alkali metals are a group column in the periodic table consisting of the chemical elements lithium Li sodium Na potassium K rubidium Rb caesium Cs and francium Fr.
Where M is an alkali metal. Click here to get PDF DOWNLOAD for all questions and answers of this chapter - CENGAGE CHEMISTRY Class 11 S-BLOCK GROUP 1 - ALKALI METALS. What is another name for the elements in group 7.
They are very reactive elements They have the lowest first ionization energies of the elements They all readily form ions with a. Excellent conductivity of electricity and heat. Which one of the following is not true about alkali metals.
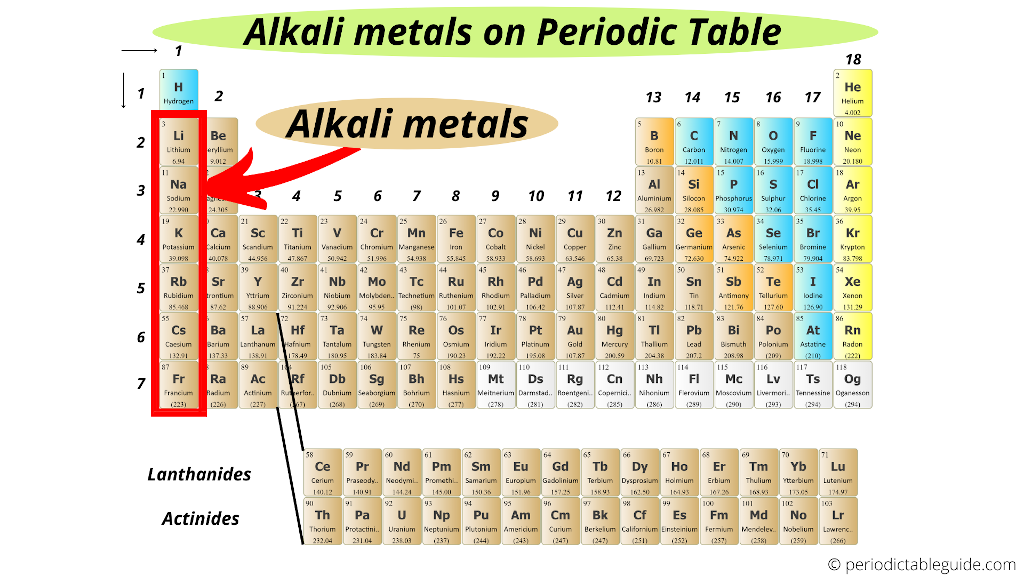
Where Are Alkali Metals Located On The Periodic Table

Which Of The Following Is Not An Alkali Metal Youtube

Alkali Metals Metallic Bonding Relatively Weak Because There Is Only One Valence Electron Meta Periodic Table Of The Elements Periodic Table Study Time Table
No comments for "Which of the Following Is Not an Alkali Metal"
Post a Comment